Nuclear reactions: Difference between revisions
imported>Mark Rust No edit summary |
imported>Meg Taylor (update) |
||
| (6 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
A combination of [[radiochemistry]] and [[radiation chemistry]] is used to study nuclear reactions such as [[nuclear fission|fission]] and [[nuclear fusion|fusion]]. Some early evidence for nuclear fission was the formation of a shortlived radioisotope of [[barium]] which was isolated from [[neutron]] irradiated [[uranium]] ( <sup>139</sup>Ba, with a half-life of 83 minutes and <sup>140</sup>Ba, with a half-life of 12.8 days, are major [[fission product]]s of uranium). At the time, it was thought that this was a new radium isotope, as it was then standard radiochemical practice to use a barium sulphate carrier precipitate to assist in the isolation of [[radium]].[http://chemcases.com/nuclear/nc-03.htm]. More recently, a combination of radiochemical methods and nuclear physics has been used to try to make new 'superheavy' elements; it is thought that islands of relative stability exist where the nuclides have half-lives of years, thus enabling weighable amounts of the new elements to be isolated. For more details of the original discovery of nuclear fission see the work of [[Otto Hahn]].<ref>Meitner L, Frisch OR (1939) Disintegration of uranium by neutrons: a new type of nuclear reaction ''Nature'' '''143''':239-240 [http://dbhs.wvusd.k12.ca.us/webdocs/Chem-History/Meitner-Fission-1939.html]</ref>. | {{subpages}} | ||
{{TOC|right}} | |||
A combination of [[radiochemistry]] and [[radiation chemistry]] is used to study nuclear reactions such as [[nuclear fission|fission]] and [[nuclear fusion|fusion]]. Some early evidence for nuclear fission was the formation of a shortlived radioisotope of [[barium]] which was isolated from [[neutron]] irradiated [[uranium]] (<sup>139</sup>Ba, with a half-life of 83 minutes and <sup>140</sup>Ba, with a half-life of 12.8 days), are major [[fission product]]s of uranium). At the time, it was thought that this was a new radium isotope, as it was then standard radiochemical practice to use a barium sulphate carrier precipitate to assist in the isolation of [[radium]].[http://chemcases.com/nuclear/nc-03.htm]. More recently, a combination of radiochemical methods and nuclear physics has been used to try to make new 'superheavy' elements; it is thought that islands of relative stability exist where the nuclides have half-lives of years, thus enabling weighable amounts of the new elements to be isolated. For more details of the original discovery of nuclear fission see the work of [[Otto Hahn]].<ref>Meitner L, Frisch OR (1939) Disintegration of uranium by neutrons: a new type of nuclear reaction ''Nature'' '''143''':239-240 [http://dbhs.wvusd.k12.ca.us/webdocs/Chem-History/Meitner-Fission-1939.html]</ref>. | |||
==Radioisotope production== | ==Radioisotope production== | ||
| Line 5: | Line 7: | ||
===Processes=== | ===Processes=== | ||
* By irradiation with '''slow neutrons''', it is possible to form neutron-rich isotopes | * By irradiation with '''slow neutrons''', it is possible to form neutron-rich isotopes that tend to decay by beta decay (i.e. by electron emission from the nuclei). For instance, irradiating <sup>59</sup>Co with neutrons forms an excited state of <sup>60</sup>Co (best written as <sup>60m</sup>Co) which decays by emitting a [[gamma ray]] to the ground state of <sup>60</sup>Co, and which in turn decays by emitting an [[electron]] to form <sup>60m</sup>Ni. The excited state of the <sup>60m</sup>Ni then decays with the emission of two gamma photons to the ground state of <sup>60</sup>Ni. As the neutron energy increases, the simple capture reactions become less important, while other reactions such as the (n,p) reaction become more important. An example is the production of [[phosphorus]]-32 by neutron irradiation of <sup>32</sup>S. The [[sulphur]] nucleus captures a neutron and emits a [[proton]] to form the radioactive phosphorus isotope ( <sup>32</sup>P). Carbon-14 is obtained in a similar manner by irradiating <sup>14</sup>N with neutrons. | ||
*A beam of '''fast moving positive particles''' can be obtained using a [[cyclotron]] or a [[linear accelerator]] (linac); up to 30MeV protons and deuterons can be obtained this way. The energies of these particles are so high that they can overcome the [[electrostatic]] barrier which opposes the entry of positive particles into the nucleus. An example of the use of the (p,n) reaction is the conversion of <sup>103</sup>Rh into <sup>103</sup>Pd, this can be done by irradiating [[rhodium]] foil with protons to form the radioactive [[palladium]] isotope. The reaction of [[beryllium]] with alpha particles is another example. While the reaction of <sup>9</sup>Be with <sup>4</sup>He<sup>2+</sup> generates <sup>12</sup>C, its most important aspect is that it generates [[neutron]]s. | *A beam of '''fast moving positive particles''' can be obtained using a [[cyclotron]] or a [[linear accelerator]] (linac); up to 30MeV protons and deuterons can be obtained this way. The energies of these particles are so high that they can overcome the [[electrostatic]] barrier which opposes the entry of positive particles into the nucleus. An example of the use of the (p,n) reaction is the conversion of <sup>103</sup>Rh into <sup>103</sup>Pd, this can be done by irradiating [[rhodium]] foil with protons to form the radioactive [[palladium]] isotope. The reaction of [[beryllium]] with alpha particles is another example. While the reaction of <sup>9</sup>Be with <sup>4</sup>He<sup>2+</sup> generates <sup>12</sup>C, its most important aspect is that it generates [[neutron]]s. | ||
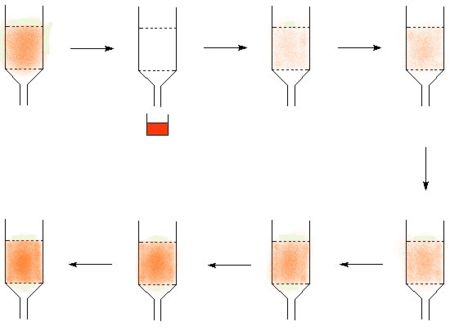
* Many isotopes can be made from a '''parent isotope''' which decays to form the desired isotope. If the parent and the product element can be chemically separated, then it is possible to create an "isotope cow". The classic isotope cow is the [[technetium cow]], many others work by the same principle. The technetium cow uses [[molybdum]]-99 absorbed on alumina, and it is "milked" by passing saline solution through it to give a solution of technetium. | * Many isotopes can be made from a '''parent isotope''' which decays to form the desired isotope. If the parent and the product element can be chemically separated, then it is possible to create an "isotope cow". The classic isotope cow is the [[technetium cow]], many others work by the same principle. The technetium cow uses [[molybdum]]-99 absorbed on alumina, and it is "milked" by passing saline solution through it to give a solution of technetium. {{Image|Growthoftcinacow.jpg|left|450px|A diagram explaining the operation of a technetium cow, the technetium, represented in red, is milked off the alumina column and then builds up again}} | ||
| Line 39: | Line 41: | ||
===Uses=== | ===Uses=== | ||
Radioactive sources have many different uses <ref>A short review of [http://www.world-nuclear.org/info/printable_information_papers/inf56print.htm the use of radioactivity in industry]</ref>. A ''sealed source'' is sealed within a container so that, in normal use, no radioactive material is lost from the source. In many sealed sources, the radioactive filling is surrounded by one or more layers of a [[corrosion]]-resistant material (such as [[stainless steel]] or [[gold]]). Alternatively, it is possible to make a source using material which holds the radioactivity in a chemically resistant and strong form without needing a metal cover. In designing sealed sources, it is common to choose a chemically stable form of the radioactive element, but for [[ | Radioactive sources have many different uses <ref>A short review of [http://www.world-nuclear.org/info/printable_information_papers/inf56print.htm the use of radioactivity in industry]</ref>. A ''sealed source'' is sealed within a container so that, in normal use, no radioactive material is lost from the source. In many sealed sources, the radioactive filling is surrounded by one or more layers of a [[corrosion]]-resistant material (such as [[stainless steel]] or [[gold]]). Alternatively, it is possible to make a source using material which holds the radioactivity in a chemically resistant and strong form without needing a metal cover. In designing sealed sources, it is common to choose a chemically stable form of the radioactive element, but for [[caesium]] radiotherapy sources it is common to use the water soluble [[chloride]], because it is impossible to obtain a high enough density of caesium in any other compound. | ||
*'''Sealed sources''' are used for radiotherapy treatment of many [[cancer]]s as well as for [[food irradiation]], [[industrial radiography]], [[nuclear gauges]] and many other applications. In medical radiotherapy, tumors can be treated either by focusing a beam of gamma rays on the area of the body that contains the tumor ([[teletherapy]]),, or by surgically placing a smaller radioactive source within or close to the tumor ([[brachytherapy]]). The aim is to confine the radiation, as far as possible, to the tumor and to spare healthy tissues in other parts of the body from its effects. | *'''Sealed sources''' are used for radiotherapy treatment of many [[cancer]]s as well as for [[food irradiation]], [[industrial radiography]], [[nuclear gauges]] and many other applications. In medical radiotherapy, tumors can be treated either by focusing a beam of gamma rays on the area of the body that contains the tumor ([[teletherapy]]),, or by surgically placing a smaller radioactive source within or close to the tumor ([[brachytherapy]]). The aim is to confine the radiation, as far as possible, to the tumor and to spare healthy tissues in other parts of the body from its effects. | ||
| Line 60: | Line 62: | ||
== References == | == References == | ||
{{reflist}} | |||
Revision as of 08:27, 12 September 2013
A combination of radiochemistry and radiation chemistry is used to study nuclear reactions such as fission and fusion. Some early evidence for nuclear fission was the formation of a shortlived radioisotope of barium which was isolated from neutron irradiated uranium (139Ba, with a half-life of 83 minutes and 140Ba, with a half-life of 12.8 days), are major fission products of uranium). At the time, it was thought that this was a new radium isotope, as it was then standard radiochemical practice to use a barium sulphate carrier precipitate to assist in the isolation of radium.[2]. More recently, a combination of radiochemical methods and nuclear physics has been used to try to make new 'superheavy' elements; it is thought that islands of relative stability exist where the nuclides have half-lives of years, thus enabling weighable amounts of the new elements to be isolated. For more details of the original discovery of nuclear fission see the work of Otto Hahn.[1].
Radioisotope production
The processes forming new isotopes (often radioactive) involve several areas of nuclear chemistry is an important subsection of this field.
Processes
- By irradiation with slow neutrons, it is possible to form neutron-rich isotopes that tend to decay by beta decay (i.e. by electron emission from the nuclei). For instance, irradiating 59Co with neutrons forms an excited state of 60Co (best written as 60mCo) which decays by emitting a gamma ray to the ground state of 60Co, and which in turn decays by emitting an electron to form 60mNi. The excited state of the 60mNi then decays with the emission of two gamma photons to the ground state of 60Ni. As the neutron energy increases, the simple capture reactions become less important, while other reactions such as the (n,p) reaction become more important. An example is the production of phosphorus-32 by neutron irradiation of 32S. The sulphur nucleus captures a neutron and emits a proton to form the radioactive phosphorus isotope ( 32P). Carbon-14 is obtained in a similar manner by irradiating 14N with neutrons.
- A beam of fast moving positive particles can be obtained using a cyclotron or a linear accelerator (linac); up to 30MeV protons and deuterons can be obtained this way. The energies of these particles are so high that they can overcome the electrostatic barrier which opposes the entry of positive particles into the nucleus. An example of the use of the (p,n) reaction is the conversion of 103Rh into 103Pd, this can be done by irradiating rhodium foil with protons to form the radioactive palladium isotope. The reaction of beryllium with alpha particles is another example. While the reaction of 9Be with 4He2+ generates 12C, its most important aspect is that it generates neutrons.
- Many isotopes can be made from a parent isotope which decays to form the desired isotope. If the parent and the product element can be chemically separated, then it is possible to create an "isotope cow". The classic isotope cow is the technetium cow, many others work by the same principle. The technetium cow uses molybdum-99 absorbed on alumina, and it is "milked" by passing saline solution through it to give a solution of technetium.
In the diagram, the technetium is represented in red, in picture two the cow is milked to make a product solution. The technetium then builds up again to allow the cow to return to the technetium loaded state where it can be milked again.
In this way, aqueous solutions of the following isotopes can be made from parent isotopes (shown in brackets)
- 68Ga (68Ge)
- 82Rb (82Sr)
- 99mTc (99Mo)
- 113mIn (113Sn)
- 188Re (188W)
- 62Cu (62Zn)
When the product isotope is a gas, the cow can be milked by allowing the product to diffuse out of a solid. An early way of making radiography sources was to milk radon from a radium source; this method was used by Marie Curie during the first World War (WWI), and was used in the USA to make Brachytherapy sources. By this method, the following isotopes can be obtained from parent isotopes (shown in brackets)
- 81mKr (81Rb)
- 222Rn (226Ra)
In some nuclear materials, new isotopes are formed by the decay of a parent isotope. For instance, the beta decay of 241Pu will form 241Am, so if a sample of plutonium which has been standing for several years is subjected to a new chemical purification, then it is possible to harvest the americium.
- 241Am (241Pu)
Uses
Radioactive sources have many different uses [2]. A sealed source is sealed within a container so that, in normal use, no radioactive material is lost from the source. In many sealed sources, the radioactive filling is surrounded by one or more layers of a corrosion-resistant material (such as stainless steel or gold). Alternatively, it is possible to make a source using material which holds the radioactivity in a chemically resistant and strong form without needing a metal cover. In designing sealed sources, it is common to choose a chemically stable form of the radioactive element, but for caesium radiotherapy sources it is common to use the water soluble chloride, because it is impossible to obtain a high enough density of caesium in any other compound.
- Sealed sources are used for radiotherapy treatment of many cancers as well as for food irradiation, industrial radiography, nuclear gauges and many other applications. In medical radiotherapy, tumors can be treated either by focusing a beam of gamma rays on the area of the body that contains the tumor (teletherapy),, or by surgically placing a smaller radioactive source within or close to the tumor (brachytherapy). The aim is to confine the radiation, as far as possible, to the tumor and to spare healthy tissues in other parts of the body from its effects.
- Open sources are used for a range of applications which include the use of tracers to study the physical operation of industrial processes, to trace the chemical mechanism by which a product forms. For instance, krypton has been used to study the underground combustion of fuels such as oil and coal.[3][4]. They are also used for some forms of radiotherapy. For example, in the treatment of thyroid cancer the patient is given a large dose of 131I. Because the iodine accumulates in the thyroid gland, the tissue of the thyroid gland (and the tummor) suffers a much higher dose of radiation than most of the body. As a result, the radioactive iodine can selectively destroy the thyroid gland and the tumor which is derived from it. Also in terminal care 89Sr is used to destroy bone tumors. [3]
Because cancer cells are more susceptible to being killed by radiation than normal, healthy cells, radiotherapy treatment can be very effective in reducing the bulk of tumors. Radiotherapy is usually accompanied by some form of chemotherapy designed to attack any remaining tumor cells.
Some radiopharmaceuticals are used for medical imaging, including many different technetium complexes [4], while radioactive 201Tl (half-life of 73 hours) is used for diagnostic purposes in nuclear medicine, particularly in stress tests used for risk stratification in patients with coronary artery disease (CAD).[5][6] This isotope of thallium can be generated using a transportable generator which is similar to the technetium cow. The generator contains lead-201 (half life 9.33 hours) which decays by electron capture to the 201Tl. The 201Pb can be produced in a cyclotron by the bombardment of thallium with protons or deuterons by the (p,3n) and (d,4n) reactions.[7]
References
- ↑ Meitner L, Frisch OR (1939) Disintegration of uranium by neutrons: a new type of nuclear reaction Nature 143:239-240 [1]
- ↑ A short review of the use of radioactivity in industry
- ↑ Volkert WA, Hoffman TJ (1999) Therapeutic radiopharmaceuticals Chem Rev 99:2269-92 PMID 11749482
- ↑ Jurisson SS, Lydon JD (1999) Potential technetium small molecule radiopharmaceuticals Chem Rev 99:2205-18 PMID 11749479
- ↑ Thallium Test from Walter Reed Army Medical Center
- ↑ Thallium Stress Test from the American Heart Association
- ↑ Thallium-201 production from Harvard Medical School's Joint Program in Nuclear Medicine