Metabolism/Citable Version: Difference between revisions
imported>Pedro Silva No edit summary |
imported>Pedro Silva No edit summary |
||
| Line 10: | Line 10: | ||
== Overview == | == Overview == | ||
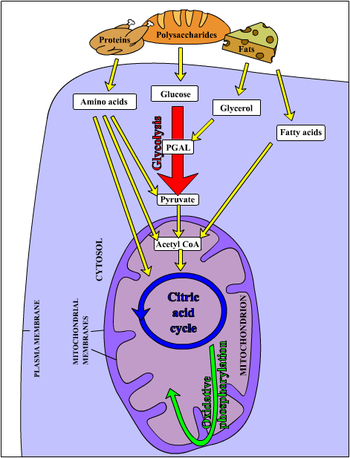
[[Enzymes]] present in [[cell]]s are able to catalyze a large variety of chemical reactions with exquisite specificity. Often, the chemical reactions needed to synthesize useful cell components involve a positive change in [[Gibbs free energy|free energy]], i.e. they are not spontaneous. In these cases, enzymes may couple the non-spontaneous reaction to a second, very spontaneous, reaction, so that the overall process is spontaneous. | |||
The spontaneous reaction most often used to drive metabolism is hydrolysis of [[ATP]] in [[ADP]] and[[phosphate]] anion. Therefore, ATP synthesis from ADP and phosphate is one of the major tasks faced by cells. | |||
According to the energy source for ATP synthesis, organisms can be classified as: | |||
*[[phototrophy|phototrophic]], which can obtain energy from light. A typical example is provided by the [[light dependent reaction]]s of photosynthesis: in these reactions, excitation of a [[photosystem]] caused by absorption of a light photon markedly lowers its redox potential. Since electron flow tends to occur from low potential species to high potential species, the excited photosystem transfers electrons to higher potential species in an electron transport chain present in the [[thylakoid]] membrane. These electrons eventually reduce NADP<sup>+</sup> to [[NADPH]]. The energy released in the electron transfer steps is used to transport H<sup>+</sup> across the thylakoid membrane, thereby creating a [[proton gradient]] across the thylakoid membrane. The energy stored in this proton gradient can be used to synthesize [[ATP]] from [[ADP]] and [[phosphate]] anion (see [[Chemiosmotic theory]]). | |||
*[[chemotrophy|chemotrophic]], which obtain energy from chemical reactions. For example, [[glucose]] can be oxidized to [[pyruvate]] through [[glycolysis]]. This process yields 2 ATP per glucose molecule, and releases 4 electrons, which reduce NAD<sup>+</sup> to [[NADH]]. Since the NAD<sup>+</sup> is a scarce molecule, the electrons present in NADH must be transferred to another molecule in order to regenerate NAD<sup>+</sup> and to allow the degradation of additional glucose molecules. NADH may donate its electrons to pyruvate (or to a pyruvate derivative), in which case a [[fermentation]] is said to occur. Alternatively, the electron acceptor may be a molecule totally unrelated to the metabolic pathway that released the electrons now present in NADH, in which case a [[respiration]] is said to occur. | |||
In the presence of NAD<sup>+</sup>, [[pyruvate dehydrogenase]] may decarboxylate pyruvate into [[acetyl-CoA]], a pivotal molecule in metabolism. [[Acetyl-CoA]] can also be formed through [[Beta-oxidation]] of [[fatty acids]] or through the catabolism of amino acids, and is oxidized to CO<sub>2</sub> through the [[Krebs cycle]]. The Krebs cycle releases 8 electrons from each acetyl-CoA molecule, which are used to reduce three NAD<sup>+</sup> to three [[NADH]] and one [[FAD]] to FADH<sub>2</sub>. The energy released in electron transfer from NADH and FADH<sub>2</sub> to oxygen (in aerobic organisms) or other electron acceptor (in organisms that perform anaerobic respiration) may be used to create a [[proton gradient]] across a membrane, and to synthesize ATP through dissipation of this gradient (see [[Chemiosmotic theory]]). | |||
Organisms can be also be cassified, according to the source of reducing power, as: | |||
*organotrophic - use organic compounds (e.g. [[glucose]]) as electron donors. | |||
*[[lithotrophy|lithotrophic]] - use inorganic compounds (e.g. Fe<sup>2+</sup>) as electron donors. | |||
== Metabolic pathways == | == Metabolic pathways == | ||
Revision as of 07:49, 2 November 2006
Metabolism (from Greek μεταβολισμός "metabolismos") is the biochemical modification of chemical compounds in living organisms and cells. Metabolism has two distinct divisions: anabolism, in which a cell uses energy and reducing power to construct complex molecules and perform other life functions such a creating cellular structure; and catabolism, in which a cell breaks down complex molecules to yield energy and reducing power. Cell metabolism involves extremely complex sequences of controlled chemical reactions called metabolic pathways.
History

The first controlled experiments in human metabolism were published by Santorio Santorio in 1614 in his book Ars de statica medecina that made him famous throughout Europe. He describes his long series of experiments in which he weighed himself in a chair suspended from a steelyard balance (see image), before and after eating, sleeping, working, sex, fasting, depriving from drinking, and excreting. He found that by far the greatest part of the food he took in was lost from the body through perspiratio insensibilis (insensible perspiration). At around the same time Jan Baptist van Helmont made the first observations regarding photosynthesis, when he discovered that plant growth required (almost) no soil nutrients. In the XVIII century, Priestley concluded that green plants use CO2 and release O2. In 1804, Nicolas de Saussure discovered that the increase in carbon content of plants (i.e. plant growth) arises from the fixation of atmospheric CO2. Between 1854 and 1864, Louis Pasteur discovered that glucose fermentation is due to microorganisms, and in 1897 Eduard Buchner proved that cell-free yeast extracts could also perform these reactions, and therefore the ability to ferment was not limited to living creatures. Subsequent investigations showed that (with a few exceptions) all living organisms metabolize glucose using the same mechanism.
Overview
Enzymes present in cells are able to catalyze a large variety of chemical reactions with exquisite specificity. Often, the chemical reactions needed to synthesize useful cell components involve a positive change in free energy, i.e. they are not spontaneous. In these cases, enzymes may couple the non-spontaneous reaction to a second, very spontaneous, reaction, so that the overall process is spontaneous. The spontaneous reaction most often used to drive metabolism is hydrolysis of ATP in ADP andphosphate anion. Therefore, ATP synthesis from ADP and phosphate is one of the major tasks faced by cells.
According to the energy source for ATP synthesis, organisms can be classified as:
- phototrophic, which can obtain energy from light. A typical example is provided by the light dependent reactions of photosynthesis: in these reactions, excitation of a photosystem caused by absorption of a light photon markedly lowers its redox potential. Since electron flow tends to occur from low potential species to high potential species, the excited photosystem transfers electrons to higher potential species in an electron transport chain present in the thylakoid membrane. These electrons eventually reduce NADP+ to NADPH. The energy released in the electron transfer steps is used to transport H+ across the thylakoid membrane, thereby creating a proton gradient across the thylakoid membrane. The energy stored in this proton gradient can be used to synthesize ATP from ADP and phosphate anion (see Chemiosmotic theory).
- chemotrophic, which obtain energy from chemical reactions. For example, glucose can be oxidized to pyruvate through glycolysis. This process yields 2 ATP per glucose molecule, and releases 4 electrons, which reduce NAD+ to NADH. Since the NAD+ is a scarce molecule, the electrons present in NADH must be transferred to another molecule in order to regenerate NAD+ and to allow the degradation of additional glucose molecules. NADH may donate its electrons to pyruvate (or to a pyruvate derivative), in which case a fermentation is said to occur. Alternatively, the electron acceptor may be a molecule totally unrelated to the metabolic pathway that released the electrons now present in NADH, in which case a respiration is said to occur.
In the presence of NAD+, pyruvate dehydrogenase may decarboxylate pyruvate into acetyl-CoA, a pivotal molecule in metabolism. Acetyl-CoA can also be formed through Beta-oxidation of fatty acids or through the catabolism of amino acids, and is oxidized to CO2 through the Krebs cycle. The Krebs cycle releases 8 electrons from each acetyl-CoA molecule, which are used to reduce three NAD+ to three NADH and one FAD to FADH2. The energy released in electron transfer from NADH and FADH2 to oxygen (in aerobic organisms) or other electron acceptor (in organisms that perform anaerobic respiration) may be used to create a proton gradient across a membrane, and to synthesize ATP through dissipation of this gradient (see Chemiosmotic theory).
Organisms can be also be cassified, according to the source of reducing power, as:
- organotrophic - use organic compounds (e.g. glucose) as electron donors.
- lithotrophic - use inorganic compounds (e.g. Fe2+) as electron donors.
Metabolic pathways
Important metabolic pathways are:
General pathways
Anabolism
Anabolic pathways that create building blocks and compounds from simple precursors:
- Glycogenesis
- Gluconeogenesis
- Porphyrin synthesis pathway
- HMG-CoA reductase pathway, leading to cholesterol and isoprenoids.
- Secondary metabolism, metabolic pathways that are not essential for growth, development or reproduction, but that usually have ecological function.
- Photosynthesis
- Light-dependent reaction (light reaction)
- Light-independent reaction (dark reaction)
- Calvin cycle
- Carbon fixation
- Glyoxylate cycle
Catabolism
- Glycolysis
- Glycogenolysis
- Citric acid cycle
- Beta-oxidation of fatty acids
Drug metabolism
Drug metabolism pathways, the modification or degradation of drugs and other xenobiotic compounds through specialized enzyme systems:
Nitrogen metabolism
Nitrogen metabolism includes the pathways for turnover and excretion of nitrogen in organisms as well as the biological processes of the biogeochemical nitrogen cycle:
- Urea cycle, important for excretion of nitrogen as urea.
- Biological nitrogen fixation
- Nitrogen assimilation
- Nitrification
- Denitrification
Other
See also
- Cell metabolism
- Metabolomics
- Metabolome
- Metabolite
- Basal metabolic rate
- Thermic effect of food
- Iron-sulfur world theory, a "metabolism first" theory of the origin of life.
- Biodegradation
- Calorimetry
- Respirometry
- Microbial metabolism
- Metabolic network modelling
- dynamic energy budget
External links
- Interactive Flow Chart of the Major Metabolic Pathways
- Metabolism, Cellular Respiration and Photosynthesis - The Virtual Library of Biochemistry and Cell Biology
- The Biochemistry of Metabolism at Rensselaer Polytechnic Institute
- Flow Chart of Metabolic Pathways at ExPASy
- Santorio Santorio's experiments
- KEGG: Kyoto Encyclopedia of Genes and Genomes