Adipocyte: Difference between revisions
imported>Gareth Leng No edit summary |
imported>David E. Volk |
||
| (35 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
The '''adipocyte''', a cell type located in [[adipose tissue]], stores excess [[fat]] and makes it available on demand to the body system for use as energy. Adipocytes also secrete signaling messengers, called ''[[adipokines]]''. Adipose tissue — which consists of other cell types besides adipocytes — operates as an endocrine organ, secreting more than 50 known adipokines.<ref>'''''Note:''''' Adipokines influence such processes as [[inflammation]], control of [[appetite]] and [[energy balance]], sensitivity to insulin, the metabolism of fats, and the [[angiogenesis|growth of new blood vessels]].</ref> | The '''adipocyte''', a cell type located in [[adipose tissue]], stores excess [[fat]] and makes it available on demand to the body system for use as energy. Adipocytes also secrete signaling messengers, called ''[[adipokines]]''. Adipose tissue—which consists of other cell types besides adipocytes—operates as an endocrine organ, secreting more than 50 known adipokines.<ref>'''''Note:''''' Adipokines influence such processes as [[inflammation]], control of [[appetite]] and [[energy balance]], sensitivity to insulin, the metabolism of fats, and the [[angiogenesis|growth of new blood vessels]].</ref><ref>Flier J ''et al.'' (1987) Severely impaired adipsin expression in genetic and acquired obesity ''Science'' 237:405-8 PMID 3299706 - Realisation that the fat cell is more than just a fat storage molecule</ref> | ||
There are two different types of adipose tissue | There are two different types of adipose tissue. ''White adipose tissue'' (WAT) is the fat storage tissue and ''[[brown adipose tissue]]'' (BAT) produces heat. BAT is sometimes called ‘baby fat’ as it is present in babies but converts to WAT in adulthood. This conversion is performed by [[mitochondrial uncoupling protein 1]] (UCP1), which is activated by the diet, in the [[mitochondria]]. Mice that are genetically made to express UCP1 in their WAT are resistant to [[obesity]]. This has sparked interest in a possible treatment for obesity: by identifying what causes UCP1 to stop converting BAT to WAT, it may be possible to find a way to prevent that, so that excess energy is released as heat rather than being stored in WAT. | ||
==Pioneering studies== | ==Pioneering studies== | ||
Fat cells were first considered to be able to sense energy demands and signal to decrease food intake in the | Fat cells were first considered to be able to sense energy demands and signal to decrease food intake in the 1950s. However, this was not confirmed until 1973, when Coleman, in animal experiments involving [[parabiosis]], provided evidence that a circulating factor existed that signalled information about the body's energy requirements. This idea gained little attention until Siiteri in 1987 examined the role of obesity in cancers of the reproductive tract and found that adipocytes secreted the hormone [[estrogen]] which is implicated in breast and [[endometrial cancer]]s. Estrogen is produced by the [[aromatase]] enzyme which is present in many tissues including adipose tissue. The production of estrogen is accelerated in obese people, as more adipose tissue means more estrogen and this is correlated with a higher incidence of cancers in the reproductive tract in obese individuals. Another prospect which supported the adipocyte as a regulator of energy stores came when [[adipsin]], a [[serine protease]], was also discovered to be secreted by adipocytes, and has since been found to be deficient in some animal models of obesity. | ||
Obesity brings | Obesity brings associated complications including cardiovascular problems, some cancers and [[diabetes mellitus type 2]] (T2DM). Cases of obesity are increasing in adults, as are cases of T2DM. The reason for the strong relationship between obesity and T2DM is still unclear, but it has been proposed that the physiology of adipocytes may hold the key. | ||
==Resistin== | ==Resistin== | ||
In 2001, an article in the ''Washington Post'' read | In 2001, an article in the ''Washington Post'' read: ‘Hormone may be key to diabetes’.<ref>''Washington Post'' Jan 18, 2001</ref> The hormone was ''resistin'', secreted from adipocytes of white adipose tissue in rodents, and it had been found to be involved in the emergence of T2DM. Resistin blood serum levels were significantly increased in cases of obesity and it was found to interfere with the actions of insulin and with glucose tolerance, causing insulin resistance in mice. This was also supported by research showing that deliberately induced diabetes, caused by provision of a high fat diet, caused obesity and elevated resistin levels in mice. These effects could be reversed by administration of a resistin antibody.<ref>Steppan CM ''et al.'' (2001) The hormone resistin links obesity to diabetes ''Nature'' 409:307-12 PMID 11201732 -First discovered resistin as a link between obesity and diabetes</ref> This discovery sparked excitement, as resistin appeared to be a candidate for the missing link between obesity and T2DM. However, studies in humans showed no significant differences between blood serum resistin levels between lean and obese people, and no differences in serum resistin levels between healthy and diabetic people. These conclusions suggest that resistin does not play a role in obesity-related diabetes and does not seem to be governed by adiposity. The differences between humans and mice may be due to differences in energy metabolism and in genetic differences between the species, as the mouse resistin gene is not identical to the human gene, only showing 59% homology.<ref>Lee JH ''et al.'' (2003) Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects ''J Clin Endocrinol Metab'' 88:4848-56 PMID 14557464</ref> | ||
More recent studies have found supernormal ''plasma'' concentrations of resistin in humans (South Koreans) with | More recent studies have found supernormal ''plasma'' concentrations of resistin in humans (South Koreans) with T2DM<ref>Youn BS ''et al.'' (2004) Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus ''J Clin Endocrinol Metab'' 89:150-6 PMID 14715842</ref> though the studies yielded no insight into the role of the higher resistin levels. They were not related to gender, or to the severity of obesity or insulin resistance. Saudi Arabians with T2DM also have increased plasma resistin levels when assessed for the presence of the [[metabolic syndrome]], an array of risk factors for cardiovascular disease (e.g., obesity in and around the abdomen; increased blood levels of lipids that predispose to atherosclerosis; impaired ability to use insulin or blood sugar; indications of [[inflammation]] in the body).<ref>Al-Daghri NM ''et al.'' (2008) Adipocytokine profile of type 2 diabetics in metabolic syndrome as defined by various criteria ''Diabetes Metab Res Rev'' [http://www3.interscience.wiley.com/cgi-bin/fulltext/114297091/HTMLSTART 24:52-8] PMID 17657721</ref> That association of high resistin levels and the metabolic syndrome has been confirmed by other investigators.<ref>Norata GD ''et al.'' (2007) Plasma resistin levels correlate with determinants of the metabolic syndrome ''Eur J Endocrinol'' 156:279-84</ref> In a Turkish study group, investigators found that one specific form of the resistin gene ([[polymorphism]]) associated with insulin resistance and obesity in patients with T2DM<ref>Duman BS ''et al.'' (2007) Association of resistin gene 3'-untranslated region EX4-44G-->A polymorphism with obesity- and insulin-related phenotypes in Turkish type 2 diabetes patients ''Rev Diabet Stud'' 4:49-55</ref> | ||
Taken together, those findings link resistin and T2DM, but shed no light on the functional role of resistin in humans. Because | Taken together, those findings link resistin and T2DM, but shed no light on the functional role of resistin in humans. Because cells involved in immunity and inflammation also produce resistin, the link between resistin and T2DM might relate to [[inflammation|inflammatory]] reactions, an area needing further investigation.<ref>Lago F ''et al.'' (2007) The emerging role of adipokines as mediators of [[inflammation]] and immune responses ''Cytokine Growth Factor Rev'' 18:313-25</ref><ref>Anderson PD ''et al.'' (2007) Innate immunity modulates adipokines in humans ''J Clin Endocrinol Metab'' 92:2272-9</ref><ref name=barnes2009>Barnes KM, Miner JL (2009) [http:/dx.doi.org/10.2174/138920309787315239 Role of resistin in insulin sensitivity in rodents and humans] ''Curr Protein Pept Sci'' 10:96-107</ref> | ||
In 879 non-diabetic humans, Reilly ''et al.'' tested for an association between plasma resistin concentrations and metabolic and inflammatory markers, including coronary artery calcification as an index of atherosclerosis, finding positive associations with inflammatory markers (soluble tumor necrosis factor-receptor-2; interleukin-6; and lipoprotein-associated phospholipase A2. They found no association with insulin resistance, but did with coronary calcification after appropriate justments for confounders. They concluded: "''Plasma resistin levels are correlated with markers of inflammation and are predictive of coronary atherosclerosis in humans, independent of CRP [C-reactive protein, a marker of inflammation]. Resistin may represent a novel link between metabolic signals, inflammation, and atherosclerosis.''"<ref>Reilly MP ''et al.'' (2005) Resistin is an inflammatory marker of atherosclerosis in humans ''Circulation'' [http://dx.doi.org/10.1161/01.CIR.0000155620.10387.43 111:932-9]</ref> | In 879 non-diabetic humans, Reilly ''et al.'' tested for an association between plasma resistin concentrations and metabolic and inflammatory markers, including coronary artery calcification as an index of atherosclerosis, finding positive associations with inflammatory markers (soluble tumor necrosis factor-receptor-2; [[interleukin-6]]; and [[lipoprotein-associated phospholipase A2]]. They found no association with insulin resistance, but did with coronary calcification after appropriate justments for confounders. They concluded: "''Plasma resistin levels are correlated with markers of inflammation and are predictive of coronary atherosclerosis in humans, independent of CRP [C-reactive protein, a marker of inflammation]. Resistin may represent a novel link between metabolic signals, inflammation, and atherosclerosis.''"<ref>Reilly MP ''et al.'' (2005) Resistin is an inflammatory marker of atherosclerosis in humans ''Circulation'' [http://dx.doi.org/10.1161/01.CIR.0000155620.10387.43 111:932-9]</ref> | ||
A recent review of the inflammatory-regulator role of resistin concludes: | |||
<blockquote> | |||
<p style="margin-left: 2.0%; margin-right: 6%; font-size: 1.0em; font-family: Gill Sans MT, Trebuchet MS;"> "Increasing evidence indicates [resistin's] important regulatory roles in various biological processes, including several inflammatory diseases. Further studies have shown that resistin in humans, in contrast to its production by adipocytes in mice, is synthesized predominantly by mononuclear cells both within and outside adipose tissue. Possible roles for resistin in obesity-related subclinical inflammation, atherosclerosis and cardiovascular disease, non-alcoholic fatty liver disease, rheumatic diseases, malignant tumors, asthma, inflammatory bowel disease, and chronic kidney disease have already been demonstrated. In addition, resistin can modulate several molecular pathways involved in metabolic, inflammatory, and autoimmune diseases. In this review, current knowledge about the functions and pathophysiological implications of resistin in different human pathologies is summarized, although there is a significant lack of firm evidence regarding the specific role resistin plays in the "orchestra" of the numerous mediators of inflammation."<ref name=filkova2009>Filkova M ''et al.'' (2009) [http://dx.doi.org/10.1016/j.clim.2009.07.013 The role of resistin as a regulator of inflammation: Implications for various human pathologies] ''Clin Immunol'' 133:157-70</ref></p> | |||
</blockquote> | |||
==Visfatin== | ==Visfatin== | ||
Another adipokine implicated in the obesity-diabetes | Another adipokine implicated in the obesity-diabetes link is ''[[visfatin]]''. Visfatin is secreted by visceral fat and has glucose-lowering effects like those of [[insulin]]. Visfatin and insulin stimulate muscle and adipose cells to take up glucose and restrain glucose release from hepatocytes.<ref>Fukuhara ''et al.'' (2005) Visfatin: a protein secreted by visceral fat that mimics the effects of insulin ''Science'' 307:426-30 - Discovery of another protein secreted from fat that may provide the link between obesity and diabetes</ref> However, visfatin is unlike insulin in that its levels are constant regardless of food intake whereas insulin levels change depending on the feeding state. Visfatin also has significantly lower intracellular levels than insulin but interestingly it acts on the insulin receptor but not competitively. This may mean that visfatin is a mimetic of insulin, but its levels are low enough so that it does not interfere with the actions of insulin. | ||
==Leptin== | ==Leptin== | ||
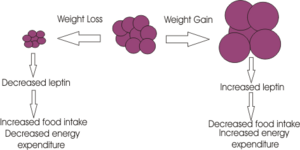
{{Image|Adipocyte.png|left|300px|}} | {{Image|Adipocyte.png|left|300px|}} | ||
Probably the biggest breakthrough for the study of appetite regulation came in 1994 when the molecular geneticist Jeffrey Friedman discovered the adiposity signal [[leptin]]. | Probably the biggest breakthrough for the study of appetite regulation came in 1994 when the molecular geneticist Jeffrey Friedman discovered the adiposity signal [[leptin]]. Using the ''ob/ob'' mice which were thought to lack a satiety signal, Friedman and colleagues found that ''ob'' codes for a gene which they called ''leptin'', from the Greek ‘leptos’ meaning thin. Mice deficient in this gene are morbidly obese and this obesity can be reversed by giving the mice leptin. The leptin receptor was found in 1995 and is a member of the [[cytokine]] receptor family.<ref>Zhang ''et al.'' (1994) Positional cloning of the mouse obese gene and its human homologue ''Nature'' 372:425-32 PMID 7984236 - these authors first identified leptin </ref> | ||
Leptin is a signalling molecule released from adipocyte cells to signal regarding adiposity levels. It is secreted into the blood, secretion proportional to body fat, where it travels to the brain causing a decrease in appetite through acting on specific neurones in the brain.<ref>Schwartz ''et al.'' (2000) Central nervous system control of food intake ''Nature'' 404:661-71 PMID 10766253 - detailing the effects of leptin on molecular pathways in the brain.</ref> | |||
Unsurprisingly, there was huge media interest in leptin as a possible treatment for obesity, and the biotechnology company Amgen paid $20 million to license leptin. However the results of clinical trials on obese people were disappointing, with very few of the participants losing weight. It appeared that most people were not obese because of a deficiency in leptin, but because they were unable to respond to it; they had more fat cells and so more circulating leptin and as a result, reduced sensitivity of the leptin receptor. The failure of the trial, however, overshadowed the fact that about 30% of the subjects lost weight and so may have low circulating levels of leptin. Potentially these people might benefit from leptin therapy. The task now though is to determine those people that have a lower circulating level of leptin. | |||
The use of leptin as a drug has proved valuable in the disease lipodystrophy and in people who have a defective leptin gene and so are constantly hungry. | |||
The weight loss caused by leptin seems to arise | Leptin inhibits food intake by acting in the appetite control centres of the brain. Leptin receptor mRNA is found primarily in the hypothalamic [arcuate nucleus]], [[ventromedial nucleus]] and [[dorsomedial hypothalamic nucleus]], regions that are known to be involved in appetite control. The weight loss caused by leptin seems to arise by two effects; | ||
1. Inhibiting appetite through its actions on the appetite-stimulating [[neuropeptide Y]] | 1. Inhibiting appetite through its actions on the appetite-stimulating [[neuropeptide Y]] (NPY) neurones and the appetite-inhibiting [[proopiomelanocortin]] (POMC) neurons, located in the hypothalamic [[arcuate nucleus]]. Leptin inhibits NPY neurons,causing a decrease in the release of the inhibitory neurotransmitter [[GABA]] (which is synthesised by the NPY neurons). This "disinhibits" the POMC neurons which increase their firing rate leading to the release of [[alpha MSH]] – a product of POMC which is a potent inhibitor of appetite. Leptin also acts directly on the POMC neurons. | ||
2. Leptin also increases energy expenditure by increasing body temperature and [[oxygen consumption]] | 2. Leptin also increases energy expenditure by increasing body temperature and [[oxygen consumption]] | ||
Leptin is synthesised in many other tissues including the [[stomach]], [[ovary]], [[placenta]] and [[liver]] and its receptors are also located in a diverse range of tissues. It has a role in many other physiological functions such as reproduction, where levels of leptin appear to dictate the commencement of [[puberty]] and in foetal development where there is a surge in leptin levels in the first week of life, which does not correspond to a decrease in food intake but is thought to be a developmental signal. During development, mice deficient in leptin have disruptions in the arcuate nucleus neural projections, but not other hypothalamic projections, and these effects can be reversed upon leptin treatment. | |||
Leptin is synthesised in many other tissues including the [[stomach]], [[ovary]], [[placenta]] and [[liver]] and its receptors are also located in a diverse range of tissues. | |||
Leptin’s also | Leptin’s also has a role in other physiological processes. For example it can be thought of as a signal informing the body when it has sufficient fat to accommodate an ‘expensive’ physiological function like reproduction. Leptin therefore appears to protect the body against starvation by only allowing energy dense processes to occur when the body is ready. Its primary role is unclear, is it a satiety signal to prevent overeating or an evolutionarily efficient signal protecting against starvation? | ||
==Adiponectin== | ==Adiponectin== | ||
[[Adiponectin]] alters insulin receptor function, diminishes the action of insulin in the liver, alters the metabolism of free fatty acids by liver cells, and protects against inflammation. Adults and children (humans) who have [[non-alcoholic fatty liver disease]] (NAFLD) show decreased plasma concentrations of adiponectin. Inasmuch as [[insulin resistance]] in the liver, and hyperinsulinemia, feature in NAFLD, those finding suggest a link between fatty liver and insulin resistance perhaps in part to reduced adiponectin. NAFLD also features elevated levels of leptin, an adipokine that reduces appetite but also interferes with insulin action in the brain.<ref>Roberts EA | [[Adiponectin]] alters insulin receptor function, diminishes the action of insulin in the liver, alters the metabolism of free fatty acids by liver cells, and protects against inflammation. Adults and children (humans) who have [[non-alcoholic fatty liver disease]] (NAFLD) show decreased plasma concentrations of adiponectin. Inasmuch as [[insulin resistance]] in the liver, and hyperinsulinemia, feature in NAFLD, those finding suggest a link between fatty liver and insulin resistance perhaps in part to reduced adiponectin. NAFLD also features elevated levels of leptin, an adipokine that reduces appetite but also interferes with insulin action in the brain.<ref>Roberts EA (2007) Pediatric nonalcoholic fatty liver disease (NAFLD): a "growing" problem?" ''J Hepatol'' 46:1133-42 PMID 17445934</ref><ref>Stefan N ''et al.'' (2006) a2-Heremans-Schmid Glycoprotein/Fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans ''Diabetes Care'' 29:853–7 PMID 16567827</ref><ref>Bugianesi E ''et al.'' (2005) Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity ''J Clin Endocrinol Metab'' 90:3498–504 PMID 15797948</ref><ref>Pagano C ''et al.'' (2005) Plasma adiponectin is decreased in nonalcoholic fatty liver disease ''Eur J Endocrinol'' 152:113–8 PMID 15762194 </ref><ref>Louthan MV ''et al.''(2005) Decreased serum adiponectin: an early event in pediatric nonalcoholic fatty liver disease ''J Pediatr'' 147:835–8</ref><ref>Gil-Campos M ''et al.'' Adiponectin, the missing link in insulin resistance and obesity ''Clin Nutr'' 23:963–74 PMID 15380884</ref> | ||
Adiponectin exhibits the following actions:<ref>Guerre-Millo M | Adiponectin exhibits the following actions:<ref>Guerre-Millo M (2007) [http://dx.doi.org/10.1016/j.diabet.2007.08.002 Adiponectin: an update] ''Diabetes Metabol'' 34:12-28</ref><ref>Fruebis J ''et al.'' (2001) Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice ''Proc Natl Acad Sci USA'' 98:2005-10 PMID 11172066 </ref><ref>Yamauchi T ''et al.'' (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. ''Nat Med'' 7:941-6</ref><ref>Berg AH ''et al.''(2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. ''Nat Med'' 7:947-953.</ref><ref>Combs TP ''et al.'' (2001) Endogenous glucose production is inhibited by the adipose-derived protein Acrp30 ''J Clin Invest'' 108:1875-81 PMID 11748271</ref><ref>Bajaj M ''et al.'' (2004) Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients ''J Clin Endocrinol Metab'' 89:200-6 PMID 14715850</ref><ref>Miyazaki Y ''et al.'' (2004) Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients ''J Clin Endocrinol Metab'' 89:4312-19 PMID 15356026</ref><ref>Bugianesi E ''et al.'' (2005) Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity ''J Clin Endocrinol Metab'' 90:3498-504 PMID 15797948 </ref> | ||
* Perhaps by increasing fatty acid oxidation, a smaller increase in plasma | * Perhaps by increasing fatty acid oxidation, a smaller increase in plasma free fatty acids occurs after a high fat meal; | ||
* By enhancing lipid metabolism, insulin sensitivity improves; | * By enhancing lipid metabolism, insulin sensitivity improves; | ||
* Reduced glucose production by the liver; | * Reduced glucose production by the liver; | ||
* Plasma concentrations associate inversely with endogenous glucose production. | * Plasma concentrations associate inversely with endogenous glucose production. | ||
In patients who have had an acute myocardial infarction, the risk of subsequent major adverse cardiovascular events is lowest in patients with the highest plasma concentrations of adiponectin. <ref>Huang SS ''et al.'' (2010) [http://www.ncbi.nlm.nih.gov/pubmed/20185863?dopt=Citation Association of adiponectin with future Cardiovascular events in patients after acute myocardial infarction | In patients who have had an acute myocardial infarction, the risk of subsequent major adverse cardiovascular events is lowest in patients with the highest plasma concentrations of adiponectin.<ref>Huang SS ''et al.'' (2010) [http://www.ncbi.nlm.nih.gov/pubmed/20185863?dopt=Citation Association of adiponectin with future Cardiovascular events in patients after acute myocardial infarction] ''J Atheroscler Thromb'' 17:295-303 PMID 20185863</ref> | ||
A complex incompletly elucidated relationship exists between adipose tissue cytokines and bone metabolism. | A complex incompletly elucidated relationship exists between adipose tissue cytokines and bone metabolism.<ref>Magni P ''et al.'' (2010) Molecular aspects of adipokine-bone interactions ''Curr Mol Med'' 10:522-32 PMID 20642443</ref> Some workers find that in postmenopausal women plasma adiponectin concentrations associate inversely with bone mineral density.<ref>Wu N ''et al.'' (2010) [http://dx.doi.org/10.1016/j.cca.2010.02.064 Relationships between serum adiponectin, leptin concentrations and bone mineral density, and bone biochemical markers in Chinese women] ''Clin Chim Acta'' 411:771-5 PMID 20184866</ref> | ||
==Table of molecules released by adipose tissue== | ==Table of molecules released by adipose tissue== | ||
| Line 71: | Line 71: | ||
|- | |- | ||
| '''[[Acylation stimulating protein]]'''<ref name=ahren2003>Ahrén B ''et al.'' (2003) [http:/dx.doi.org/10.1038/sj.ijo.0802369 Acylation stimulating protein stimulates insulin secretion | | '''[[Acylation stimulating protein]]'''<ref name=ahren2003>Ahrén B ''et al.'' (2003) [http:/dx.doi.org/10.1038/sj.ijo.0802369 Acylation stimulating protein stimulates insulin secretion] ''Int J Obesity'' 27:1037–43 | ||
*From | *From abstract: Acylation stimulating protein (ASP) is a hormone produced by adipocytes and is of importance for the storage of energy as fat….ASP augments glucose-stimulated insulin secretion through a direct action on the islet beta cells….Stimulation of insulin secretion by ASP in vivo results in augmented glucose disposal. n on the islet beta cells.</ref> <ref name=schrauwen>Schrauwen P ''et al.'' (2005) [http://dx.doi.org/10.1038/sj.ijo.0802949 Acylation-stimulating protein: effect of acute exercise and endurance training] ''Int J Obesity'' 29:632–638. | ||
*From Abstract: Acylation-stimulating protein (ASP) is an adipocyte-derived protein that contributes to fatty acid clearance….Regular exercise training improves fatty acid handling….Short-term endurance training reduces baseline ASP levels….These data fit with the hypothesis that reduced ASP levels indicate improved ASP sensitivity</ref> | *'''<u>From the Abstract:</u>''' "Acylation-stimulating protein (ASP) is an adipocyte-derived protein that contributes to fatty acid clearance….Regular exercise training improves fatty acid handling….Short-term endurance training reduces baseline ASP levels….These data fit with the hypothesis that reduced ASP levels indicate improved ASP sensitivity"</ref> | ||
|<center>'''+ '''</center> | |<center>'''+ '''</center> | ||
| Line 79: | Line 79: | ||
|- | |- | ||
| '''[[Adiponectin]]'''<ref name=millo2008>Guerre-Millo M. (2008) Adiponectin: an update | | '''[[Adiponectin]]'''<ref name=millo2008>Guerre-Millo M. (2008) Adiponectin: an update ''Diabetes Metab'' 34:12-18 PMID 18069030</ref> | ||
| <center>'''++ '''</center> | | <center>'''++ '''</center> | ||
| <center>'''+'''</center> | | <center>'''+'''</center> | ||
|- | |- | ||
| '''[[Angiotensinogen]] '''<ref name=karlsson1998>Karlsson C ''et al.'' (1998) [http://jcem.endojournals.org/cgi/content/full/83/11/3925 Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II | | '''[[Angiotensinogen]] '''<ref name=karlsson1998>Karlsson C ''et al.'' (1998) [http://jcem.endojournals.org/cgi/content/full/83/11/3925 Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II] ''J Clin Endocrinol Metab'' 83:3925-9 PMID 9814470 | ||
*'''<u>From the Abstract:</u>''' Angiotensin II regulates blood pressure and may affect adipogenesis and adipocyte metabolism. Angiotensin II is produced by cleavage of angiotensinogen by renin and angiotensin-converting enzyme in the circulation. In addition, angiotensin II may be produced in various tissues by enzymes of the renin-angiotensin system (RAS) or the nonrenin-angiotensin system (NRAS)….We conclude that human adipose tissue expresses angiotensinogen and enzymes of RAS and NRAS. This opens the possibility that angiotensinogen-derived peptides, produced in adipose tissue itself, may affect adipogenesis and play a role in the pathogenesis of obesity.</ref> <ref name=muller1998>Muller-Wieland D ''et al.'' | *'''<u>From the Abstract:</u>''' "Angiotensin II regulates blood pressure and may affect adipogenesis and adipocyte metabolism. Angiotensin II is produced by cleavage of angiotensinogen by renin and angiotensin-converting enzyme in the circulation. In addition, angiotensin II may be produced in various tissues by enzymes of the renin-angiotensin system (RAS) or the nonrenin-angiotensin system (NRAS)….We conclude that human adipose tissue expresses angiotensinogen and enzymes of RAS and NRAS. This opens the possibility that angiotensinogen-derived peptides, produced in adipose tissue itself, may affect adipogenesis and play a role in the pathogenesis of obesity."</ref> <ref name=muller1998>Muller-Wieland D ''et al.'' (1998) [http://www.springerlink.com/content/xpeh2nbh7795t06m/?p=fa23a870b2c04907928e395d167d2359&pi=15 Metabolic syndrome and hypertension: pathophysiology and molecular basis of insulin resistance] ''Basic Res Cardiol'' 93 Suppl 2:131-4 PMID 9833175 | ||
*'''<u>From the Abstract:</u>'''Several recent studies indicate that type 2 diabetes, arterial hypertension, lipid disorders as well as visceral obesity are coronary risk factors which might belong to a syndrome which is caused by decreased insulin sensitivity with compensatory hyperinsulinaemia. More than 50% of patients with essential hypertension have some degree of insulin resistance, but in contrast to dyslipoproteinaemia and glucose intolerance the causal relation between insulin resistance and elevated arterial blood pressure appears not to be as evident. One explanation is that the link between blood pressure and insulin sensitivity might be mainly related to concomitant obesity. Accordingly, obesity can be associated with an increased activity of the sympathetic nervous system, elevated plasma levels of the vasoconstrictor endothelin-1, and decreased insulin-induced endothelium-dependent vasodilation. Furthermore, adipocytes can secrete vasogenic peptides, such as angiotensinogen. Since insulin resistance is a polygenic disorder, the two basic genetic approaches we follow is to identify genetic defects of insulin action in cells of patients with inherited syndromes of insulin resistance and to characterize molecular mechanisms of insulin regulated gene expression. The results show that insulin can affect the expression rate of various genes, e.g. involved in cholesterol and fatty acid metabolism, by modulating the activity of transcription factors coupled to the MAP kinase cascade and that a genetic postreceptor defect in these intracellular signaling pathways might have a pleiotropic effect on cell metabolism and clinical phenotype.</ref> <ref name=giacchetti2000>Giacchetti G ''et al.'' (2000) [http://www.ncbi.nlm.nih.gov/pubmed/10997636?dopt=Citation Gene expression of angiotensinogen in adipose tissue of obese patients.] ''Int J Obes Relat Metab Disord'' 24 Suppl 2:S142-3 PMID 10997636. | *'''<u>From the Abstract:</u>'''"Several recent studies indicate that type 2 diabetes, arterial hypertension, lipid disorders as well as visceral obesity are coronary risk factors which might belong to a syndrome which is caused by decreased insulin sensitivity with compensatory hyperinsulinaemia. More than 50% of patients with essential hypertension have some degree of insulin resistance, but in contrast to dyslipoproteinaemia and glucose intolerance the causal relation between insulin resistance and elevated arterial blood pressure appears not to be as evident. One explanation is that the link between blood pressure and insulin sensitivity might be mainly related to concomitant obesity. Accordingly, obesity can be associated with an increased activity of the sympathetic nervous system, elevated plasma levels of the vasoconstrictor endothelin-1, and decreased insulin-induced endothelium-dependent vasodilation. Furthermore, adipocytes can secrete vasogenic peptides, such as angiotensinogen. Since insulin resistance is a polygenic disorder, the two basic genetic approaches we follow is to identify genetic defects of insulin action in cells of patients with inherited syndromes of insulin resistance and to characterize molecular mechanisms of insulin regulated gene expression. The results show that insulin can affect the expression rate of various genes, e.g. involved in cholesterol and fatty acid metabolism, by modulating the activity of transcription factors coupled to the MAP kinase cascade and that a genetic postreceptor defect in these intracellular signaling pathways might have a pleiotropic effect on cell metabolism and clinical phenotype."</ref> <ref name=giacchetti2000>Giacchetti G ''et al.'' (2000) [http://www.ncbi.nlm.nih.gov/pubmed/10997636?dopt=Citation Gene expression of angiotensinogen in adipose tissue of obese patients.] ''Int J Obes Relat Metab Disord'' 24 Suppl 2:S142-3 PMID 10997636. | ||
*'''<u>From the Abstract:</u>''' Recently, the genes of components of the renin-angiotensin system (RAS), namely angiotensinogen (AGT), angiotensin converting enzyme and angiotensin II receptor have been described in adipose tissue. In animal models the angiotensinogen in adipose tissue has been implicated in the pathogenesis of metabolic alterations and hypertension associated with obesity…. [The] data suggest that angiotensinogen may be determinant of fat distribution and may be involved in the plurimetabolic syndrome of central obesity.</ref> <ref name=bulcao2006>Bulcao C ''et al.'' (2006) [http://www.ingentaconnect.com/content/ben/cdr/2006/00000002/00000001/art00004?token=004d1972cacf449b339412f415d48772562453a7b2f7b24422d253048296a7c2849266d656cd8 The new adipose tissue and adipocytokines.] ''Curr Diabetes Rev'' 2:19-28 PMID 18220614 | *'''<u>From the Abstract:</u>''' "Recently, the genes of components of the renin-angiotensin system (RAS), namely angiotensinogen (AGT), angiotensin converting enzyme and angiotensin II receptor have been described in adipose tissue. In animal models the angiotensinogen in adipose tissue has been implicated in the pathogenesis of metabolic alterations and hypertension associated with obesity…. [The] data suggest that angiotensinogen may be determinant of fat distribution and may be involved in the plurimetabolic syndrome of central obesity."</ref> <ref name=bulcao2006>Bulcao C ''et al.'' (2006) [http://www.ingentaconnect.com/content/ben/cdr/2006/00000002/00000001/art00004?token=004d1972cacf449b339412f415d48772562453a7b2f7b24422d253048296a7c2849266d656cd8 The new adipose tissue and adipocytokines.] ''Curr Diabetes Rev'' 2:19-28 PMID 18220614 | ||
*'''<u>From the Abstract:</u>''' Obesity is a well-known risk factor for the development of insulin resistance, type 2 diabetes, dyslipidemia, hypertension, and cardiovascular disease. Rather than the total amount of fat, central distribution of adipose tissue is very important in the pathophysiology of this constellation of abnormalities termed metabolic syndrome. Adipose tissue, regarded only as an energy storage organ until the last decade, is now known as the biggest endocrine organ of the human body. This tissue secretes a number of substances--adipocytokines--with multiple functions in metabolic profile and immunological process. Therefore, excessive fat mass may trigger metabolic and hemostatic disturbances as well as CVD. Adipocytokines may act locally or distally as inflammatory, immune or hormonal signalers. In this review we discuss visceral obesity, the potential mechanisms by which it would be related to insulin resistance, methods for its assessment and focus on the main adipocytokines expressed and secreted by the adipose tissue. | *'''<u>From the Abstract:</u>''' "Obesity is a well-known risk factor for the development of insulin resistance, type 2 diabetes, dyslipidemia, hypertension, and cardiovascular disease. Rather than the total amount of fat, central distribution of adipose tissue is very important in the pathophysiology of this constellation of abnormalities termed metabolic syndrome. Adipose tissue, regarded only as an energy storage organ until the last decade, is now known as the biggest endocrine organ of the human body. This tissue secretes a number of substances--adipocytokines--with multiple functions in metabolic profile and immunological process. Therefore, excessive fat mass may trigger metabolic and hemostatic disturbances as well as CVD. Adipocytokines may act locally or distally as inflammatory, immune or hormonal signalers. In this review we discuss visceral obesity, the potential mechanisms by which it would be related to insulin resistance, methods for its assessment and focus on the main adipocytokines expressed and secreted by the adipose tissue."</ref> | ||
| <center>'''++ '''</center> | | <center>'''++ '''</center> | ||
| Line 169: | Line 169: | ||
|- | |- | ||
| colspan="3" | <font color="#000066">'''Adapted from Garrutti ''et al.''<ref name=garrutti2008>Garrutti G ''et al.'' (2008) [http://www.ncbi.nlm.nih.gov/pubmed/18568142?dopt=Citation Neuroendocrine deregulation of food intake, adipose tissue and the gastrointestinal system in obesity and metabolic syndrome | | colspan="3" | <font color="#000066">'''Adapted from Garrutti ''et al.''<ref name=garrutti2008>Garrutti G ''et al.'' (2008) [http://www.ncbi.nlm.nih.gov/pubmed/18568142?dopt=Citation Neuroendocrine deregulation of food intake, adipose tissue and the gastrointestinal system in obesity and metabolic syndrome] ''J Gastrointestin Liver Dis'' 17:193-8 PMID 18568142</ref> and modified by Citizendium editors'''</font> | ||
|} | |} | ||
{{-}} | {{-}} | ||
==Other genes of interest== | |||
Not all of the genes of interest in adipocytes encode for the production of secreted proteins. For example, [[adiponutrin]] was first identified as a gene highly expressed in adipocytes. Obesity is associated with elevated levels of adiponutrin mRNA in adipose tissue (and in liver) in humans, and so is a potential target for therapies to manage obesity. Adiponutrin/PNPLA3 is a member of the patatin-like phospholipase family, and is strongly associated with membranes and with lipid droplets. but its exact biological function is unclear. A key feature of adiponutrin is that its expression in adipose tissue is nutritionally regulated, decreasing after fasting and strongly up-regulated by feeding. The induction of expression by a meal is rapid, and depends on the macronutrient composition of the meal: induction is most marked after meals that contain high sucrose and high protein, less so on a high lipid diet. This pattern of regulation mirrors that of lipogenic genes, suggesting that adiponutrin might be involved in regulating lipogenesis or lipolysis.<ref>Rae-Whitcombe SM ''et al.''(2010) Regulation of the promoter region of the human adiponutrin/PNPLA3 gene by glucose and insulin ''Biochem Biophys Res Commun'' 402:767-72 PMID 21036152</ref>. Hobbs and coworkers<ref>{{cite journal | authors = S Romeo, J Kozlitina, C Xing, A Pertsemlidis1, D Cox, LA Pennacchio, E Boerwinkle, JC Cohen & HH Hobb | journal = Nature Genetics | volume = 40 | pages = 1461-1465 | year = 2008 | title = Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. | doi = doi:10.1038/ng.257}}</ref> found that a particular adiponutrin gene variant, which encodes for an I148 to M148 protein mutation, correlates well (P = 5.9 x 10<sup>-10</sup>) with hepatic fat levels, [[nonalcoholic fatty liver disease]] ([[steatosis]]) and [[steatic hepatitis]]. The relative mutation rates are Hispanics > European Americans > African Americans. They also found that another [[allele]] encoding S453I mutation was associated with lower hepatic fat content in African Americans. | |||
==Notes== | ==Notes== | ||
| Line 177: | Line 180: | ||
<references /> | <references /> | ||
</div> | </div> | ||
Revision as of 09:57, 18 February 2011
The adipocyte, a cell type located in adipose tissue, stores excess fat and makes it available on demand to the body system for use as energy. Adipocytes also secrete signaling messengers, called adipokines. Adipose tissue—which consists of other cell types besides adipocytes—operates as an endocrine organ, secreting more than 50 known adipokines.[1][2]
There are two different types of adipose tissue. White adipose tissue (WAT) is the fat storage tissue and brown adipose tissue (BAT) produces heat. BAT is sometimes called ‘baby fat’ as it is present in babies but converts to WAT in adulthood. This conversion is performed by mitochondrial uncoupling protein 1 (UCP1), which is activated by the diet, in the mitochondria. Mice that are genetically made to express UCP1 in their WAT are resistant to obesity. This has sparked interest in a possible treatment for obesity: by identifying what causes UCP1 to stop converting BAT to WAT, it may be possible to find a way to prevent that, so that excess energy is released as heat rather than being stored in WAT.
Pioneering studies
Fat cells were first considered to be able to sense energy demands and signal to decrease food intake in the 1950s. However, this was not confirmed until 1973, when Coleman, in animal experiments involving parabiosis, provided evidence that a circulating factor existed that signalled information about the body's energy requirements. This idea gained little attention until Siiteri in 1987 examined the role of obesity in cancers of the reproductive tract and found that adipocytes secreted the hormone estrogen which is implicated in breast and endometrial cancers. Estrogen is produced by the aromatase enzyme which is present in many tissues including adipose tissue. The production of estrogen is accelerated in obese people, as more adipose tissue means more estrogen and this is correlated with a higher incidence of cancers in the reproductive tract in obese individuals. Another prospect which supported the adipocyte as a regulator of energy stores came when adipsin, a serine protease, was also discovered to be secreted by adipocytes, and has since been found to be deficient in some animal models of obesity.
Obesity brings associated complications including cardiovascular problems, some cancers and diabetes mellitus type 2 (T2DM). Cases of obesity are increasing in adults, as are cases of T2DM. The reason for the strong relationship between obesity and T2DM is still unclear, but it has been proposed that the physiology of adipocytes may hold the key.
Resistin
In 2001, an article in the Washington Post read: ‘Hormone may be key to diabetes’.[3] The hormone was resistin, secreted from adipocytes of white adipose tissue in rodents, and it had been found to be involved in the emergence of T2DM. Resistin blood serum levels were significantly increased in cases of obesity and it was found to interfere with the actions of insulin and with glucose tolerance, causing insulin resistance in mice. This was also supported by research showing that deliberately induced diabetes, caused by provision of a high fat diet, caused obesity and elevated resistin levels in mice. These effects could be reversed by administration of a resistin antibody.[4] This discovery sparked excitement, as resistin appeared to be a candidate for the missing link between obesity and T2DM. However, studies in humans showed no significant differences between blood serum resistin levels between lean and obese people, and no differences in serum resistin levels between healthy and diabetic people. These conclusions suggest that resistin does not play a role in obesity-related diabetes and does not seem to be governed by adiposity. The differences between humans and mice may be due to differences in energy metabolism and in genetic differences between the species, as the mouse resistin gene is not identical to the human gene, only showing 59% homology.[5]
More recent studies have found supernormal plasma concentrations of resistin in humans (South Koreans) with T2DM[6] though the studies yielded no insight into the role of the higher resistin levels. They were not related to gender, or to the severity of obesity or insulin resistance. Saudi Arabians with T2DM also have increased plasma resistin levels when assessed for the presence of the metabolic syndrome, an array of risk factors for cardiovascular disease (e.g., obesity in and around the abdomen; increased blood levels of lipids that predispose to atherosclerosis; impaired ability to use insulin or blood sugar; indications of inflammation in the body).[7] That association of high resistin levels and the metabolic syndrome has been confirmed by other investigators.[8] In a Turkish study group, investigators found that one specific form of the resistin gene (polymorphism) associated with insulin resistance and obesity in patients with T2DM[9]
Taken together, those findings link resistin and T2DM, but shed no light on the functional role of resistin in humans. Because cells involved in immunity and inflammation also produce resistin, the link between resistin and T2DM might relate to inflammatory reactions, an area needing further investigation.[10][11][12]
In 879 non-diabetic humans, Reilly et al. tested for an association between plasma resistin concentrations and metabolic and inflammatory markers, including coronary artery calcification as an index of atherosclerosis, finding positive associations with inflammatory markers (soluble tumor necrosis factor-receptor-2; interleukin-6; and lipoprotein-associated phospholipase A2. They found no association with insulin resistance, but did with coronary calcification after appropriate justments for confounders. They concluded: "Plasma resistin levels are correlated with markers of inflammation and are predictive of coronary atherosclerosis in humans, independent of CRP [C-reactive protein, a marker of inflammation]. Resistin may represent a novel link between metabolic signals, inflammation, and atherosclerosis."[13]
A recent review of the inflammatory-regulator role of resistin concludes:
"Increasing evidence indicates [resistin's] important regulatory roles in various biological processes, including several inflammatory diseases. Further studies have shown that resistin in humans, in contrast to its production by adipocytes in mice, is synthesized predominantly by mononuclear cells both within and outside adipose tissue. Possible roles for resistin in obesity-related subclinical inflammation, atherosclerosis and cardiovascular disease, non-alcoholic fatty liver disease, rheumatic diseases, malignant tumors, asthma, inflammatory bowel disease, and chronic kidney disease have already been demonstrated. In addition, resistin can modulate several molecular pathways involved in metabolic, inflammatory, and autoimmune diseases. In this review, current knowledge about the functions and pathophysiological implications of resistin in different human pathologies is summarized, although there is a significant lack of firm evidence regarding the specific role resistin plays in the "orchestra" of the numerous mediators of inflammation."[14]
Visfatin
Another adipokine implicated in the obesity-diabetes link is visfatin. Visfatin is secreted by visceral fat and has glucose-lowering effects like those of insulin. Visfatin and insulin stimulate muscle and adipose cells to take up glucose and restrain glucose release from hepatocytes.[15] However, visfatin is unlike insulin in that its levels are constant regardless of food intake whereas insulin levels change depending on the feeding state. Visfatin also has significantly lower intracellular levels than insulin but interestingly it acts on the insulin receptor but not competitively. This may mean that visfatin is a mimetic of insulin, but its levels are low enough so that it does not interfere with the actions of insulin.
Leptin
Probably the biggest breakthrough for the study of appetite regulation came in 1994 when the molecular geneticist Jeffrey Friedman discovered the adiposity signal leptin. Using the ob/ob mice which were thought to lack a satiety signal, Friedman and colleagues found that ob codes for a gene which they called leptin, from the Greek ‘leptos’ meaning thin. Mice deficient in this gene are morbidly obese and this obesity can be reversed by giving the mice leptin. The leptin receptor was found in 1995 and is a member of the cytokine receptor family.[16]
Leptin is a signalling molecule released from adipocyte cells to signal regarding adiposity levels. It is secreted into the blood, secretion proportional to body fat, where it travels to the brain causing a decrease in appetite through acting on specific neurones in the brain.[17]
Unsurprisingly, there was huge media interest in leptin as a possible treatment for obesity, and the biotechnology company Amgen paid $20 million to license leptin. However the results of clinical trials on obese people were disappointing, with very few of the participants losing weight. It appeared that most people were not obese because of a deficiency in leptin, but because they were unable to respond to it; they had more fat cells and so more circulating leptin and as a result, reduced sensitivity of the leptin receptor. The failure of the trial, however, overshadowed the fact that about 30% of the subjects lost weight and so may have low circulating levels of leptin. Potentially these people might benefit from leptin therapy. The task now though is to determine those people that have a lower circulating level of leptin.
The use of leptin as a drug has proved valuable in the disease lipodystrophy and in people who have a defective leptin gene and so are constantly hungry.
Leptin inhibits food intake by acting in the appetite control centres of the brain. Leptin receptor mRNA is found primarily in the hypothalamic [arcuate nucleus]], ventromedial nucleus and dorsomedial hypothalamic nucleus, regions that are known to be involved in appetite control. The weight loss caused by leptin seems to arise by two effects;
1. Inhibiting appetite through its actions on the appetite-stimulating neuropeptide Y (NPY) neurones and the appetite-inhibiting proopiomelanocortin (POMC) neurons, located in the hypothalamic arcuate nucleus. Leptin inhibits NPY neurons,causing a decrease in the release of the inhibitory neurotransmitter GABA (which is synthesised by the NPY neurons). This "disinhibits" the POMC neurons which increase their firing rate leading to the release of alpha MSH – a product of POMC which is a potent inhibitor of appetite. Leptin also acts directly on the POMC neurons.
2. Leptin also increases energy expenditure by increasing body temperature and oxygen consumption
Leptin is synthesised in many other tissues including the stomach, ovary, placenta and liver and its receptors are also located in a diverse range of tissues. It has a role in many other physiological functions such as reproduction, where levels of leptin appear to dictate the commencement of puberty and in foetal development where there is a surge in leptin levels in the first week of life, which does not correspond to a decrease in food intake but is thought to be a developmental signal. During development, mice deficient in leptin have disruptions in the arcuate nucleus neural projections, but not other hypothalamic projections, and these effects can be reversed upon leptin treatment.
Leptin’s also has a role in other physiological processes. For example it can be thought of as a signal informing the body when it has sufficient fat to accommodate an ‘expensive’ physiological function like reproduction. Leptin therefore appears to protect the body against starvation by only allowing energy dense processes to occur when the body is ready. Its primary role is unclear, is it a satiety signal to prevent overeating or an evolutionarily efficient signal protecting against starvation?
Adiponectin
Adiponectin alters insulin receptor function, diminishes the action of insulin in the liver, alters the metabolism of free fatty acids by liver cells, and protects against inflammation. Adults and children (humans) who have non-alcoholic fatty liver disease (NAFLD) show decreased plasma concentrations of adiponectin. Inasmuch as insulin resistance in the liver, and hyperinsulinemia, feature in NAFLD, those finding suggest a link between fatty liver and insulin resistance perhaps in part to reduced adiponectin. NAFLD also features elevated levels of leptin, an adipokine that reduces appetite but also interferes with insulin action in the brain.[18][19][20][21][22][23]
Adiponectin exhibits the following actions:[24][25][26][27][28][29][30][31]
- Perhaps by increasing fatty acid oxidation, a smaller increase in plasma free fatty acids occurs after a high fat meal;
- By enhancing lipid metabolism, insulin sensitivity improves;
- Reduced glucose production by the liver;
- Plasma concentrations associate inversely with endogenous glucose production.
In patients who have had an acute myocardial infarction, the risk of subsequent major adverse cardiovascular events is lowest in patients with the highest plasma concentrations of adiponectin.[32]
A complex incompletly elucidated relationship exists between adipose tissue cytokines and bone metabolism.[33] Some workers find that in postmenopausal women plasma adiponectin concentrations associate inversely with bone mineral density.[34]
Table of molecules released by adipose tissue
Adipose Tissue |
Adipose Tissue | |
| Acylation stimulating protein[35] [36] | ||
| Adiponectin[37] | ||
| Angiotensinogen [38] [39] [40] [41] | ||
| Atrial Natriuretic Peptide | ||
| Cholesteryl-ester transferase | ||
| Estrogens | ||
| Free Fatty Acids/Glycerol | ||
| IGF-binding protein 3 (IGFBP3) | ||
| Insulin-like growth factor-I | ||
| Interleukin-6 | ||
| Leptin | ||
| Lipoprotein lipase | ||
| Monobutyrin | ||
| Plasminogen Activator Inhibitor - factor 1 | ||
| Resistin | ||
| Retinol binding protein-4 | ||
| Tumor necrosis factor-a | ||
| Visfatin | ||
| Adapted from Garrutti et al.[42] and modified by Citizendium editors | ||
Other genes of interest
Not all of the genes of interest in adipocytes encode for the production of secreted proteins. For example, adiponutrin was first identified as a gene highly expressed in adipocytes. Obesity is associated with elevated levels of adiponutrin mRNA in adipose tissue (and in liver) in humans, and so is a potential target for therapies to manage obesity. Adiponutrin/PNPLA3 is a member of the patatin-like phospholipase family, and is strongly associated with membranes and with lipid droplets. but its exact biological function is unclear. A key feature of adiponutrin is that its expression in adipose tissue is nutritionally regulated, decreasing after fasting and strongly up-regulated by feeding. The induction of expression by a meal is rapid, and depends on the macronutrient composition of the meal: induction is most marked after meals that contain high sucrose and high protein, less so on a high lipid diet. This pattern of regulation mirrors that of lipogenic genes, suggesting that adiponutrin might be involved in regulating lipogenesis or lipolysis.[43]. Hobbs and coworkers[44] found that a particular adiponutrin gene variant, which encodes for an I148 to M148 protein mutation, correlates well (P = 5.9 x 10-10) with hepatic fat levels, nonalcoholic fatty liver disease (steatosis) and steatic hepatitis. The relative mutation rates are Hispanics > European Americans > African Americans. They also found that another allele encoding S453I mutation was associated with lower hepatic fat content in African Americans.
Notes
- ↑ Note: Adipokines influence such processes as inflammation, control of appetite and energy balance, sensitivity to insulin, the metabolism of fats, and the growth of new blood vessels.
- ↑ Flier J et al. (1987) Severely impaired adipsin expression in genetic and acquired obesity Science 237:405-8 PMID 3299706 - Realisation that the fat cell is more than just a fat storage molecule
- ↑ Washington Post Jan 18, 2001
- ↑ Steppan CM et al. (2001) The hormone resistin links obesity to diabetes Nature 409:307-12 PMID 11201732 -First discovered resistin as a link between obesity and diabetes
- ↑ Lee JH et al. (2003) Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects J Clin Endocrinol Metab 88:4848-56 PMID 14557464
- ↑ Youn BS et al. (2004) Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus J Clin Endocrinol Metab 89:150-6 PMID 14715842
- ↑ Al-Daghri NM et al. (2008) Adipocytokine profile of type 2 diabetics in metabolic syndrome as defined by various criteria Diabetes Metab Res Rev 24:52-8 PMID 17657721
- ↑ Norata GD et al. (2007) Plasma resistin levels correlate with determinants of the metabolic syndrome Eur J Endocrinol 156:279-84
- ↑ Duman BS et al. (2007) Association of resistin gene 3'-untranslated region EX4-44G-->A polymorphism with obesity- and insulin-related phenotypes in Turkish type 2 diabetes patients Rev Diabet Stud 4:49-55
- ↑ Lago F et al. (2007) The emerging role of adipokines as mediators of inflammation and immune responses Cytokine Growth Factor Rev 18:313-25
- ↑ Anderson PD et al. (2007) Innate immunity modulates adipokines in humans J Clin Endocrinol Metab 92:2272-9
- ↑ Barnes KM, Miner JL (2009) [http:/dx.doi.org/10.2174/138920309787315239 Role of resistin in insulin sensitivity in rodents and humans] Curr Protein Pept Sci 10:96-107
- ↑ Reilly MP et al. (2005) Resistin is an inflammatory marker of atherosclerosis in humans Circulation 111:932-9
- ↑ Filkova M et al. (2009) The role of resistin as a regulator of inflammation: Implications for various human pathologies Clin Immunol 133:157-70
- ↑ Fukuhara et al. (2005) Visfatin: a protein secreted by visceral fat that mimics the effects of insulin Science 307:426-30 - Discovery of another protein secreted from fat that may provide the link between obesity and diabetes
- ↑ Zhang et al. (1994) Positional cloning of the mouse obese gene and its human homologue Nature 372:425-32 PMID 7984236 - these authors first identified leptin
- ↑ Schwartz et al. (2000) Central nervous system control of food intake Nature 404:661-71 PMID 10766253 - detailing the effects of leptin on molecular pathways in the brain.
- ↑ Roberts EA (2007) Pediatric nonalcoholic fatty liver disease (NAFLD): a "growing" problem?" J Hepatol 46:1133-42 PMID 17445934
- ↑ Stefan N et al. (2006) a2-Heremans-Schmid Glycoprotein/Fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans Diabetes Care 29:853–7 PMID 16567827
- ↑ Bugianesi E et al. (2005) Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity J Clin Endocrinol Metab 90:3498–504 PMID 15797948
- ↑ Pagano C et al. (2005) Plasma adiponectin is decreased in nonalcoholic fatty liver disease Eur J Endocrinol 152:113–8 PMID 15762194
- ↑ Louthan MV et al.(2005) Decreased serum adiponectin: an early event in pediatric nonalcoholic fatty liver disease J Pediatr 147:835–8
- ↑ Gil-Campos M et al. Adiponectin, the missing link in insulin resistance and obesity Clin Nutr 23:963–74 PMID 15380884
- ↑ Guerre-Millo M (2007) Adiponectin: an update Diabetes Metabol 34:12-28
- ↑ Fruebis J et al. (2001) Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice Proc Natl Acad Sci USA 98:2005-10 PMID 11172066
- ↑ Yamauchi T et al. (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941-6
- ↑ Berg AH et al.(2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947-953.
- ↑ Combs TP et al. (2001) Endogenous glucose production is inhibited by the adipose-derived protein Acrp30 J Clin Invest 108:1875-81 PMID 11748271
- ↑ Bajaj M et al. (2004) Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients J Clin Endocrinol Metab 89:200-6 PMID 14715850
- ↑ Miyazaki Y et al. (2004) Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients J Clin Endocrinol Metab 89:4312-19 PMID 15356026
- ↑ Bugianesi E et al. (2005) Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity J Clin Endocrinol Metab 90:3498-504 PMID 15797948
- ↑ Huang SS et al. (2010) Association of adiponectin with future Cardiovascular events in patients after acute myocardial infarction J Atheroscler Thromb 17:295-303 PMID 20185863
- ↑ Magni P et al. (2010) Molecular aspects of adipokine-bone interactions Curr Mol Med 10:522-32 PMID 20642443
- ↑ Wu N et al. (2010) Relationships between serum adiponectin, leptin concentrations and bone mineral density, and bone biochemical markers in Chinese women Clin Chim Acta 411:771-5 PMID 20184866
- ↑ Ahrén B et al. (2003) [http:/dx.doi.org/10.1038/sj.ijo.0802369 Acylation stimulating protein stimulates insulin secretion] Int J Obesity 27:1037–43
- From abstract: Acylation stimulating protein (ASP) is a hormone produced by adipocytes and is of importance for the storage of energy as fat….ASP augments glucose-stimulated insulin secretion through a direct action on the islet beta cells….Stimulation of insulin secretion by ASP in vivo results in augmented glucose disposal. n on the islet beta cells.
- ↑ Schrauwen P et al. (2005) Acylation-stimulating protein: effect of acute exercise and endurance training Int J Obesity 29:632–638.
- From the Abstract: "Acylation-stimulating protein (ASP) is an adipocyte-derived protein that contributes to fatty acid clearance….Regular exercise training improves fatty acid handling….Short-term endurance training reduces baseline ASP levels….These data fit with the hypothesis that reduced ASP levels indicate improved ASP sensitivity"
- ↑ Guerre-Millo M. (2008) Adiponectin: an update Diabetes Metab 34:12-18 PMID 18069030
- ↑ Karlsson C et al. (1998) Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II J Clin Endocrinol Metab 83:3925-9 PMID 9814470
- From the Abstract: "Angiotensin II regulates blood pressure and may affect adipogenesis and adipocyte metabolism. Angiotensin II is produced by cleavage of angiotensinogen by renin and angiotensin-converting enzyme in the circulation. In addition, angiotensin II may be produced in various tissues by enzymes of the renin-angiotensin system (RAS) or the nonrenin-angiotensin system (NRAS)….We conclude that human adipose tissue expresses angiotensinogen and enzymes of RAS and NRAS. This opens the possibility that angiotensinogen-derived peptides, produced in adipose tissue itself, may affect adipogenesis and play a role in the pathogenesis of obesity."
- ↑ Muller-Wieland D et al. (1998) Metabolic syndrome and hypertension: pathophysiology and molecular basis of insulin resistance Basic Res Cardiol 93 Suppl 2:131-4 PMID 9833175
- From the Abstract:"Several recent studies indicate that type 2 diabetes, arterial hypertension, lipid disorders as well as visceral obesity are coronary risk factors which might belong to a syndrome which is caused by decreased insulin sensitivity with compensatory hyperinsulinaemia. More than 50% of patients with essential hypertension have some degree of insulin resistance, but in contrast to dyslipoproteinaemia and glucose intolerance the causal relation between insulin resistance and elevated arterial blood pressure appears not to be as evident. One explanation is that the link between blood pressure and insulin sensitivity might be mainly related to concomitant obesity. Accordingly, obesity can be associated with an increased activity of the sympathetic nervous system, elevated plasma levels of the vasoconstrictor endothelin-1, and decreased insulin-induced endothelium-dependent vasodilation. Furthermore, adipocytes can secrete vasogenic peptides, such as angiotensinogen. Since insulin resistance is a polygenic disorder, the two basic genetic approaches we follow is to identify genetic defects of insulin action in cells of patients with inherited syndromes of insulin resistance and to characterize molecular mechanisms of insulin regulated gene expression. The results show that insulin can affect the expression rate of various genes, e.g. involved in cholesterol and fatty acid metabolism, by modulating the activity of transcription factors coupled to the MAP kinase cascade and that a genetic postreceptor defect in these intracellular signaling pathways might have a pleiotropic effect on cell metabolism and clinical phenotype."
- ↑ Giacchetti G et al. (2000) Gene expression of angiotensinogen in adipose tissue of obese patients. Int J Obes Relat Metab Disord 24 Suppl 2:S142-3 PMID 10997636.
- From the Abstract: "Recently, the genes of components of the renin-angiotensin system (RAS), namely angiotensinogen (AGT), angiotensin converting enzyme and angiotensin II receptor have been described in adipose tissue. In animal models the angiotensinogen in adipose tissue has been implicated in the pathogenesis of metabolic alterations and hypertension associated with obesity…. [The] data suggest that angiotensinogen may be determinant of fat distribution and may be involved in the plurimetabolic syndrome of central obesity."
- ↑ Bulcao C et al. (2006) The new adipose tissue and adipocytokines. Curr Diabetes Rev 2:19-28 PMID 18220614
- From the Abstract: "Obesity is a well-known risk factor for the development of insulin resistance, type 2 diabetes, dyslipidemia, hypertension, and cardiovascular disease. Rather than the total amount of fat, central distribution of adipose tissue is very important in the pathophysiology of this constellation of abnormalities termed metabolic syndrome. Adipose tissue, regarded only as an energy storage organ until the last decade, is now known as the biggest endocrine organ of the human body. This tissue secretes a number of substances--adipocytokines--with multiple functions in metabolic profile and immunological process. Therefore, excessive fat mass may trigger metabolic and hemostatic disturbances as well as CVD. Adipocytokines may act locally or distally as inflammatory, immune or hormonal signalers. In this review we discuss visceral obesity, the potential mechanisms by which it would be related to insulin resistance, methods for its assessment and focus on the main adipocytokines expressed and secreted by the adipose tissue."
- ↑ Garrutti G et al. (2008) Neuroendocrine deregulation of food intake, adipose tissue and the gastrointestinal system in obesity and metabolic syndrome J Gastrointestin Liver Dis 17:193-8 PMID 18568142
- ↑ Rae-Whitcombe SM et al.(2010) Regulation of the promoter region of the human adiponutrin/PNPLA3 gene by glucose and insulin Biochem Biophys Res Commun 402:767-72 PMID 21036152
- ↑ (2008) "Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease.". Nature Genetics 40: 1461-1465. DOI:doi:10.1038/ng.257. Research Blogging.